|
by Kaitlyn Lew, 20' Scientists have been fighting cancer in a continuous battle. However, over the past few decades, researchers have discovered several breakthrough treatments. Of particular interest within the Nobel Prize Committee was immunotherapy. On October 1, 2018, the Nobel Prize of Medicine was awarded jointly to James P. Allison of University of California, Berkeley, and Tasuku Honjo of Kyoto University for their work on inhibition of negative immune regulation as cancer therapy. [1] So, what is immunotherapy? 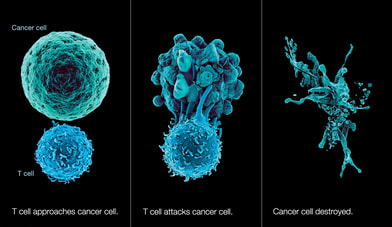 Immunotherapy is a type of cancer treatment that recruits the dynamic and highly specific immune system to fight diseases, especially cancer. Besides immunotherapy, cancer may be treated through surgery, chemotherapy, and radiation therapy. [2] However, unlike the other treatments, immunotherapy is a biological therapy that can mark certain cancer cells for the immune cells to find and destroy. Immunotherapy has several classes, such as adoptive cell transfer of T cells and bispecific antibodies. Nobel Laureates Allison and Honjo both worked on proteins that inhibit the “brakes” on the immune system, thereby allowing the immune cells to be unleashed onto tumors. Dr. Honjo discovered Programmed Cell Death Protein 1 (PD-1), which is expressed on the surface of T-cells that also operates as a brake through a different mechanism. [3] Meanwhile, Allison was one of the first scientists to study the T-cell protein CTLA-4 as a brake on T-cells. Previously, pharmaceutical companies had doubted that CTLA-4 was effective as a cancer treatment. Nevertheless, Allison’s lab continued to experiment with CTLA-4. In an experiment published in 1996, Allison’s team showed that blocking CLTA-4 reduces inhibitory signals to enhance the antitumor response. They received breakthrough results from experiments on patients with advanced melanoma, the first-ever observed. According to Brown University Professor Amanda Jamieson, a colleague of Allison, “He was so excited about that experiment that he was showing everyone.… The first CT scan had metastasize[d] everywhere, and then in the second one, it was all gone”. [4] Due to the excitement, they even performed a second trial over Christmas break. In the experiment, “[m]ice with cancer had been cured by treatment with the antibodies that inhibit the brake and unlock antitumor T-cell activity”.[1] T-cell activation not only requires the antigen to be presented on MHC molecules, but it also needs additional co-stimulation through the interaction of CD28 on T cells with primary ligands B7-1 (CD80) and B7-2 (CD86) on the surface of specialized antigen-presenting cells (APCs). [5] In the early 1980s, Allison was one of the first to identify the T cell receptor that binds to antigen and functions as the T cell’s ignition switch. Then in 1992, he showed that CD28 functions as the T cell’s “gas pedal”. Finally in 1995, he uncovered the T cell's brakes. [6] Jamieson explained, “When [Allison] started [the experiments], he really pushed it from basic research at Berkeley. People critisize basic research, especially in mouse studies, and he wanted to figure out how T cells work. Later, he noted: " Now that [we've] figured it out, it can be used to treat disease. He pushed it from basic research all the way into the clinic… to the treatment”. [4] Such promising results may create a new collection of weapons that unleashes the immune system to better battle tumors and win the war against cancer. Jamieson states, “There are going to be more cancers [that are] more complicated to treat with immunotherapy. I think in the future, cancer treatment will become more personalized to the individual tumor to look at the unique signature”. [4] Personalized medicine requires time and money, but it is the ultimate goal to effectively winning the individual battles over cancer. Works Cited
0 Comments
Leave a Reply. |