|
By Olivia Woodford-Berry, '19 In the age of stem cell engineering and genetic research, groundbreaking gene therapies, treatments that reprograms a patient’s own cells to behave differently, has captured the imagination of scientists and laymen alike. Though this therapeutic space signals a potential turning point in several difficult disease areas, the scientific and regulatory landscape surrounding these therapeutics has made the translation of ideas from bench to bedside a relatively slow process. Still, since the first gene therapy was approved in 2017, this field has made meaningful breakthroughs in diseases with dismal outlooks, and it continues to expand the boundaries of medicine. 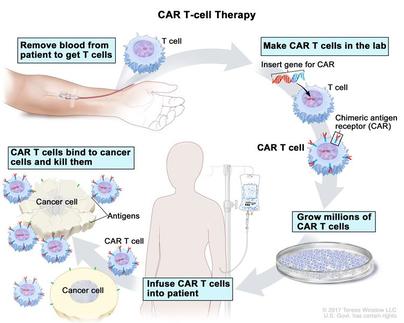 In August of 2017, the United States’ Food and Drug Administration (FDA) approved the first gene therapy for use in the United States for treatment of Acute Lymphoblastic Leukemia (ALL). While this disease has a five year five-year fatality rate a little over thirty percent, this treatment targets a segment of the population with an even more dismal outllook. The therapy, Kymriah, is an ALL treatment for patients who who have also experienced relapses or whose cancer cells are insensitive to typical therapies. The patient’s T-cells, which are immune cells and a type of white blood cell, are collected and genetically modified to express specific receptors that direct the immune system toward leukemia cells with certain surface proteins. Once altered, the T-cells are injected back into the patient with the expectation that the enhanced immune system will be better equipped to target the disease on its own. [1] Later that same year, the FDA approved two more gene therapies. The second treatment approved, Yescarta, targets cancers through a similar mechanism as Kymriah. Yescarta acts by reprogramming a patient’s T-cells to better recognize non-Hodgkin lymphoma (NHL), which also has a five-year fatality rate close to thirty percent . [2] The third approved treatment, Voretigene Neparvovec, is for the rare eye disease retinal dystrophy. While there are over two hundred genes that can cause this inherited condition, this treatment is approved for those whose condition is due to a mutation in the gene RPE65. [3] Since these three pioneers in the gene therapy space, several more treatments have reached the United States market. Currently, there are 16 gene and cellular therapies approved by the FDA, though there will almost certainly be more in the years ahead. Looking forward, this area of research is a quickly growing and exciting sector for therapeutics for inherited diseases and cancer. [4] Sources:
1 Comment
Charlotte Brown
1/21/2019 07:29:36 pm
Thank you for sharing Amazing!
Reply
Leave a Reply. |
