|
by Olivia Woodford-Berry, 19' While it is widely accepted germline stem continuously regenerate sperm populations in mammalian males, the existence of ovarian stem cells (OSCs), or lack thereof, was long viewed as a closed book within the world of mammalian research. Since 1951, the prevailing dogma, set by Dr. Solly Zuckerman, has asserted that neo-oogenesis (egg formation) in mammals occurs neither postnatally beyond a few days nor after damage to the existing egg population. [1] That is to say, women are born with a set number of germline cells that will only decline over their lifetimes. Indeed, this data would be corroborated by later studies showing that DNA synthesis does not occur in adult ovaries. [2] However, this dogma was seriously challenged for the first time in 2004, when Tilly’s group published a paper blatantly refuting previous claims, arguing that oocyte regeneration occurs in mice. This controversial study marked the beginning a new, promising line of research surrounding female germline regeneration and reproductive health. 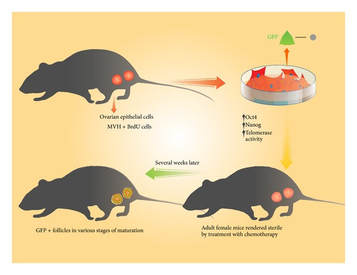 Tilly’s group maintained that oocyte regeneration is prevalent in mice through the use of experiments comparing rates of oocyte degradation (atresia) and testing genetically-engineered mice. Tilly’s group claimed that given the rate of degradation of oocytes, regeneration must occur because the germline population observed is higher than expected. Additionally, chemical treatments, previously established as a method to knockout spermatogenesis (sperm regeneration), caused a significant decrease in ovarian follicle number. [3] Since this change was long-lasting and similar to that seen in experiments on testis, researchers speculated that it may be due to a loss of regenerative capabilities and not merely a result of damage to an existing, stable oocyte population. Lastly, in a final set of experiments testing the existence of regenerative cells in mouse ovaries, mice genetically engineered to express GFP (green fluorescent protein), were grafted with wild-type (normal) mice ovaries. After three weeks, researchers saw follicle-enclosed, GFP-positive oocytes in the wild-type ovary, confirming the mouse’s ability to repopulate the oocyte environment. This data, combined with previous studies showing the ability of stem cells to migrate upon introduction to host, suggest that OSCs exist in the post-natal mouse ovary. While Tilly’s work polarized the scientific community, it has led to new and exciting body of work surrounding ovarian regeneration and therapeutic insights for women suffering from conditions such as primary ovarian insufficiency (POI). Following this study, several groups confirmed the presence of OSCs in mice, and some even showed similar patterns in prosimian primates, a much closer evolutionary relative of humans. [4][5] However, explanations surrounding this phenomenon and our capacities to compare data from different studies continue to be contested. Irrespective of the for mentioned debate, declining ovarian function remains a life-changing factor for every woman. There are many theories currently as to why, if there are OSCs in adult women, humans experience a decline in fertility during menopause. [6] Whether this problem is rooted in the deterioration of the OSC environment, the stem cell niche, or the body’s inability to activate quiescent (dormant) OSCs under natural physiological conditions, further insight into these areas could potentially lead to break throughs in fertility treatments and post-menopausal health. Sources:
0 Comments
Leave a Reply. |
