|
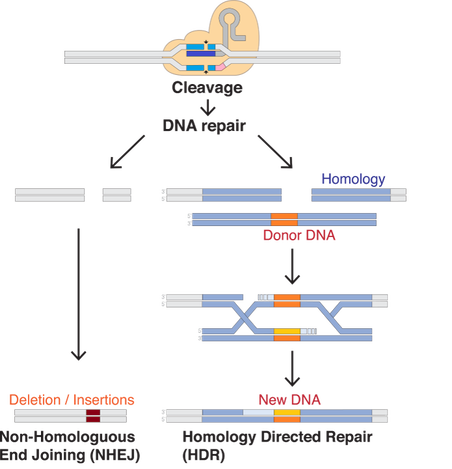
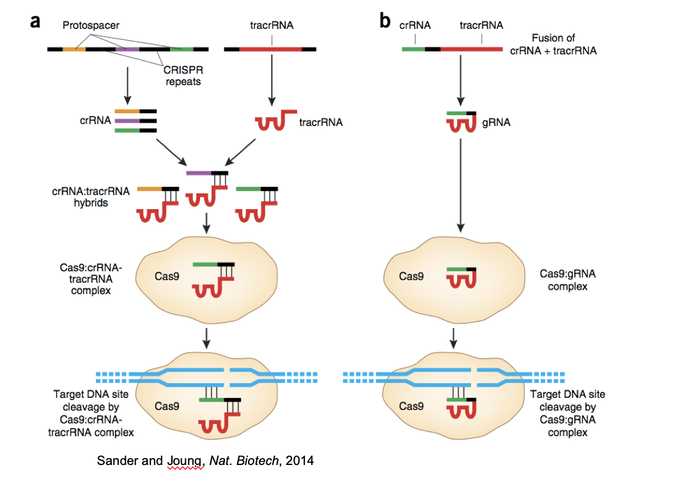
Written by: Casey Chan ‘23 Edited by: Priya Bhanot ‘23 Jennifer Doudna and Emmanuelle Charpentier made history when they received a Nobel Prize in Chemistry on October 7, 2020 for their work on the CRISPR-Cas9 system. Their landmark discovery affects fields such as medicine, agriculture, and clinical research. The CRISPR-Cas9 system has already been used in agriculture to produce corn crops that have larger and heavier kernels and therefore higher yields. Although CRISPR-Cas9 is not yet used clinically in medicine, studies are being performed to better target the tool and to prevent off-target effects. In medical trials, this tool is used as a possible treatment for diseases such as sickle-cell anemia, HIV, and cancer.One benefit of the CRISPR-Cas9 system is that it allows for precision of genome alteration. It can be used not only to remove gene function but to also add scientifically relevant sequences to different genomes. The CRISPR-Cas9 system was inspired by the immune systems of bacteria that contain regions of repeated genetic sequences interspersed with shorter genetic sequences, or “spacers,” whose function was initially unknown. The “spacers” were discovered to be phage, or virus, DNA that were captured by the bacterial cells during previous infections. These specific DNA sequences are important because they are used along with proteins in the bacteria to target and destroy invading virus DNA. This effectively provides a system of protection for the bacteria against viral infection. Emannuelle Charpentier and Jennifer Doudna demonstrated how this mechanism works in organisms such as Streptococcus pyogenes. Charpentier, Doudna, and other researchers, including Feng Zhang from MIT, have extended practical applications, such as destroying disease DNA, for this mechanism to research in other organisms. CRISPR refers to the “clustered regularly interspaced short palindromic repeats” that encode for guide RNA. Guide RNA, or the RNA that is used to bind to virus DNA in bacteria, is combined with tracrRNA, discovered by Emmanuelle Charpentier, to create an RNA complex. This RNA complex in turn attaches to CAS, or a CRISPR-associated sequence protein. The guide RNA then recognizes specific sequences of interest, and CAS proteins are responsible for cleaving, or destroying, the target sequence. The systems observed in bacteria can be practically applied to model systems and other organisms. Scientists have even optimized the process by combining the tracrRNA and the guide RNA to make a “pre-made” RNA complex that can be inserted into the genome of an organism. (More information about CRISPR-Cas9 mechanisms and applications can be found in Chris Shin’s article, “CRISPR: Mechanisms and Applications”) In the words of Professor Robert Reenan, a genetics researcher at Brown University who works with flies as model systems for neurodegenerative diseases, “this technology has allowed for more creativity with our research. In genetics, people will probably make more mutations in a very smart way.” Since CRISPR is relatively cheap and fast, its specificity allows for precise and efficient gene editing. The CRISPR-Cas9 technology is practical because the guide RNA can bind to a specific sequence, and the Cas9 can cleave the genetic target. This makes the system both efficient and very specific. After the CAS protein cleaves this sequence of interest, researchers can study the effects of DNA repair after such a dramatic change in the genome. Studies are performed on non-homologous end joining, in which the ends of two cleaved DNA fragments are repaired randomly in the cell. This method of repair often leads to disappearance of normal gene function through mutations that insert or delete genetic information. These mutations are interesting to study, especially in genes that are thought to be essential. CRISPR-Cas9 can also be used to study homology-directed repair, in which another genetic sequence is inserted along with the CRISPR-Cas9 system. The donor sequence is incorporated into the cleaved DNA, which allows geneticists to determine the effects of a new sequence incorporated into an important genetic region. Many biology professors at Brown use this technology in their own work. Professor Robert Reenan is a geneticist who focuses mostly on fly, Drosophila melanogaster, genomes. His research centers around neurodegenerative diseases in fly models. He has used CRISPR to quickly remove genes that are thought to be involved in ALS, Lou Gehrig’s disease, and fronto-temporal dementia. According to Professor Reenan, this precise method of editing is sometimes convenient compared to decades-old processes such as forward genetics, which involves the tedious study of random physical mutations. Forward genetics is a useful tool, but in certain situations it can be time consuming and expensive. Professor Reenan’s lab has successfully inserted human genes of interest, for example ALS genes, into fly model organisms in order to determine their potential effects on the fly phenotype. In the ALS mutants, the motor neurons die, leading to fly death. Professor Reenan estimates that he has used CRISPR-Cas9 to study 8-10 different disease-causing mutations in the fly model system. Since CRISPR is so cheap and fast in flies, this technology increases the opportunities to try different mutations. One example of a disease-causing mutation is in TDP43, a protein thought to be related to ALS that does not have a fully defined function. Professor Reenan’s team successfully knocked out, or deactivated, the TDP43 gene in flies, which caused the flies to have a profound locomotor defect. After adding in a human TDP43 sequence, the flies returned back to their normal state. The benefits of CRISPR-Cas9 are that this system can be used in many organisms, and it allows for more specificity of targeting because it is RNA-programmable. In other words, by inserting an RNA sequence, the Cas9 protein can target a seemingly infinite number of different genes. Professor Reenan mentions that organisms that have not been used before can now be tested using this new technology, for example ferrets. In one experiment, ferrets were used in place of the common model organism, mice, because of the higher similarity between ferret and human brains. By using CRISPR-Cas9, a more ideal organism can be targeted for researching human processes. For example, naked mole rats, which are not a common model organism, can be used for aging and cancer research. Professor Reenan mentioned that another benefit of the CRISPR-Cas9 tool is that we can provide a repair template for genes that are broken by Cas9. For example, a copy of a modified gene may be incorporated into a fly genome after cleavage by Cas9. Professor Reenan worked in a neighboring lab to Jennifer Doudna when he attended Harvard Medical School. Even during this time, he believed that her pioneering point of view was a hallmark of her career. Dr. Doudna’s projects included self-replicating RNA molecules, and she tried to create life in a cell system. According to Professor Reenan, Doudna is one of the most creative people whom he has met, and her innovation has provided others with new opportunities in their work. Doudna and Charpentier are the first two women to share the Nobel Prize in Chemistry without a male collaborator also listed on the award. Their discovery has opened the possibilities for genetic studies, and created opportunities that were previously believed to be science fiction. CRISPR-Cas9 allows for the potential alteration of human genes to eliminate diseases, the creation of hardier plants, the eradication of pathogens, and more. Doudna and Charpentier continue to foster ideas about the uses of CRISPR-Cas9 in collaboration with others in different fields. Their discovery has been a huge landmark in the field of gene-editing technology, and the extent of its applications is still yet to be seen. Works Cited:
[Image Citation] Mariuswalter (2017). File:DNA Repair After CRISPR-Cas9 cut. JPG. Retrieved 2020, from https://commons.wikimedia.org/wiki/File:DNA_Repair_after_CRISPR-Cas9_cut.svg. [Image Citation] Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotechnology [Internet]. 2014 [Cited 2020 Nov 20], 32, 349. DOI: https://doi.org/10.1038/nbt.2842.
0 Comments
Leave a Reply. |