|
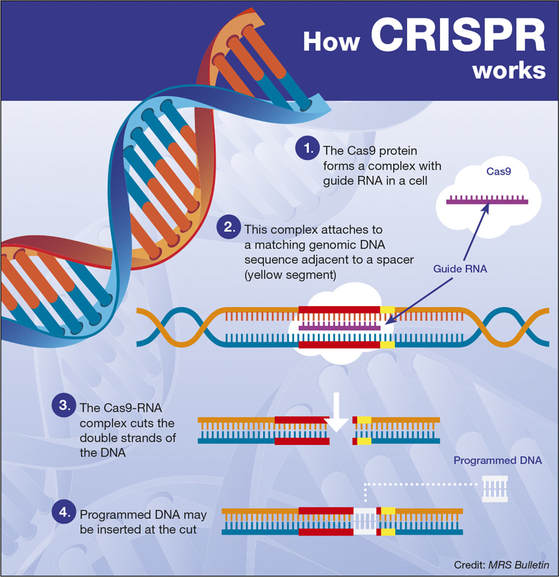
by Mitchell Yeary '19 For a lot of people, genetically engineering humans are a possibility only in Gattica. Yet there are more and more technologies coming out that put us closer to that reality. One of the most recent technologies, CRISPR, emerged a few years ago as an improved way to deactivate certain genes in cells, or create “knock-out” lines (a line of cells with certain genes that don’t function). Here, I will briefly explain CRISPR and its different applications as a genomic engineering technology. Starting with the basics, CRISPR-Cas9 as a potential genome editing system was put forth four or five years ago and was quickly leveraged so that we could start to target specific genes in the genome. There are two parts to this system. There first part is the CRISPR portion, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, essentially the type of DNA sequence that is recognized by the second part, a protein. When used as a genome editing technology, guide RNAs, which can bind to DNA, are built so that they match a section of whatever gene is being edited, and these are the CRISPR portion of the technology. The other necessary part of the guide RNA, is a loop on the end that forms beacon for Cas9, which is recruited to the site. Cas9, the second element in the system, ends up binding and then cutting the portion of the DNA that the guide RNA bound to in the beginning. By using guide RNA to selecting critical points in a gene, researchers can introduce mutations, and disable different genes. Since then, it has been engineered to be able to activate genes by inserting sequences of DNA that bypass normal gene regulation and activate specific genes. This development was laid out in a paper, “Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex” by Konermann et al. published in 2015. In this paper, he talks about how if you create a deactivated version of Cas9, so that it binds but does not cut, you can have it activate different genes.
All genes are controlled by elements in the genome called promoters. These are sequences of DNA that are designed to attract the molecular machinery responsible for transcribing DNA (the process of copying them into a form that can used to make proteins). In Konermann’s system, the guide RNA binds close to a promoter, and allow deactivated Cas9 (dCas9) to bind. The dCas9 has been modified to have a parts that bind to transcription factors, the molecular elements that turn on and off promoters. By recruiting the right transcription factors, the dCas9 can activate the promoter, which in turn activates the gene. This form of genetic manipulation has allowed us to study the effects of certain genes on different cell types. For example, it has allowed us to figure out that there is a certain gene we can activate that will cause skin cells to turn into neurons. This type of understanding of molecular genetics has wide implications in medicine, bring us closer to regrowing neurons from skins cells that can reverse paralysis, as well as paving the way towards being able to create personalized organs from scratch. Indeed, a recent article in Scientific America discuss one of the first clinical applications of CRISPR, where scientists used CRISPR-Cas9 to fix a heart defect by correcting a mutation in an embryo's genome. This is the beginning of many such treatments that will continue to be developed, and personalized medicine will become more common as we continue to improve our ability to engineering genetics. References: Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015 Jan 29;517(7536):583.
0 Comments
Leave a Reply. |