|
by Olivia Woodford-Berry '19 Though the term is greatly ambiguous in today’s charged political culture, the growth of the “healthcare crisis” is at the forefront of both American and international politics. Antibiotics, although incredibly valuable, have consistently held a key niche in this discussion. Through overuse, these have begun to become less effective [1]. According to studies published in Infection Control and Hospital Epidemiology and Internal Medicine Journal, 20-50% of all antibiotics prescribed in the United States are unnecessary [2][3]. On a global scale, the rise of antibiotic-resistant bacteria has led to severe repercussions for public health and economic stability within the healthcare system. 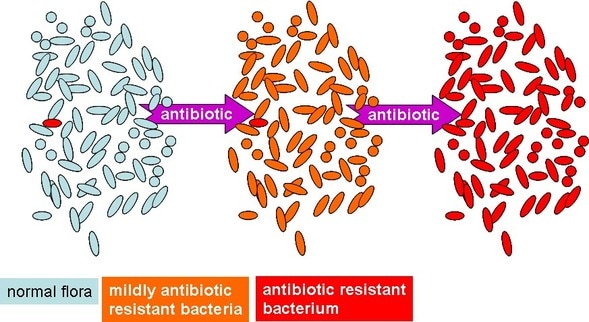 Furthermore, this issue has led to a decline in the number of clinically-usable drugs [4]. According to the Centers for Disease Control, over two million people are infected with antibiotic-resistant organisms [5], which can result in greater risks of complications, prolonged hospital visits, or death [4]. In part, this has contributed not only to the loss of human life, but also to the increasing cost of healthcare in the United States. In order to create more effective treatments, curtail rising medical costs, and constrain the prevalence of antibiotic-resistant microbes, a more focused approach to patient treatment may be in order. Personalized medicine, though often discussed with a connotation of science fiction, may be a part of the solution. Beyond the more controversial topics such as prenatal genetic screening or universal DNA sequencing, personalized medicine also refers to the more focused application of commonly used drugs. Antimicrobial stewardship refers to coordination between doctors and pharmacists to create more personalized treatments for patients’ infections. Specifically, it calls for the prescription of antimicrobials based on the individual patient’s condition and blood cultures, as opposed to the exhaustive use of common antibiotics. In a study published in the Journal of Infection, researchers examined the effects of this methodology on patients with antibiotic-resistant bloodstream infections. Subjects who were treated with rapid diagnostics and antimicrobial stewardship showed a decrease in duration of hospitalization and time in the intensive care unit compared to control groups. Furthermore, the mean hospital costs for each survivor in the experimental group reduced by nearly 30,000 dollars [4]. This methodology was shown to be more effective in terms of patient care, as intervention was a significant predictor of survival; this technique also reduced the prevalence of antibiotic resistance. Improved use of antibiotics is important in both patient safety and public health, and new approaches to antibiotic treatments are beginning to come to fruition beyond (controlled) lab settings. Programs involving antibiotic stewardship are becoming more common in hospitals. California, to take things one step further, recently instituted a law that requires hospitals to implement such programs. Still, in order for these practices to become mainstream, hospitals across the country will need greater access to highly educated drug specialists, a relatively small group of people. Still, biotechnology as a field is growing exponentially. While blanket prescriptions of antibiotics are still relatively common, widespread change in how antibiotics are handled throughout national and international healthcare systems may be on the horizon. Work Cited:
1. Huttner A, Harbarth S, Carlet J, et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrobial resistance and infection control. Nov 18 2013;2(1):31. 2. Camins BC, King MD, Wells JB, et al. Impact of an antimicrobial utilization program on antimicrobial use at a large teaching hospital: a randomized controlled trial. Infection control and hospital epidemiology :the official journal of the Society of Hospital Epidemiologists of America. Oct 2009;30(10):931-938. 3. Ingram PR, Seet JM, Budgeon CA, Murray R. Point-prevalence study of inappropriate antibiotic use at a tertiary Australian hospital. Internal medicine journal. Jun 2012;42(6):719-721. 4. Perez, Katherine K., Randall J. Olsen, William L. Musick, Patricia L. Cernoch, James R. Davis, Leif E. Peterson, and James M. Musser. "Integrating Rapid Diagnostics and Antimicrobial Stewardship Improves Outcomes in Patients with Antibiotic-resistant Gram-negative Bacteremia." Journal of Infection 69.3 (2014): 216-25. Web. 5. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013 Atlanta, GA: CDC;2013.
3 Comments
Susan Berry
3/18/2017 12:54:33 am
Olivia, great blog. Thank you. I was not aware of all this knowledge.
Reply
Kerry Rothschild
3/21/2017 11:26:21 am
I agree with your insights and wonder if at somepoint in the fututre we will look back and wonder that this wasteful and abusive approach was used at all once we had the diagnostic tools to be more specific. The costs of running basic tests before perscribing solutions, for example,cultures to determine which bacteria to target, DNA to determine how best an iduvidual's unique genes can optimize potential to be cured, and titers to determine if a innoculation is needed (another common-place overused and abused treatment) are seen as too great when compared to the cheap price of antibiotics and vaccines. That is, however, short sighted esspecially when considering the costs to the entire population and body of medicine when these solutions no longer work or create other problems because of needless over use.
Reply
3/22/2017 03:53:58 pm
Olivia--I hope you will add me to your blog mailing list. The Broad Institute is doing work in the area cited in your most recent post. I'll send you a separate email about this.--Cynthia
Reply
Leave a Reply. |
