|
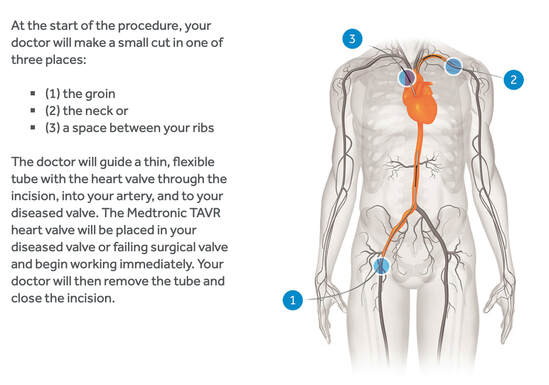
By Malika Ramani '21 Edited by Jess Sevetson Traditional open-heart surgery is both invasive and dangerous, and yet for many patients, it has been the go-to option for valve replacement. Two recent large clinical trials, however, have proven that a far less invasive – albeit “daring” procedure – may lead to better outcomes for a wide range of patients with cardiovascular complications. The procedure in question is known as “transcatheter aortic valve replacement;” TAVR, for short. In drastic contrast to a surgery that involves opening up the entire chest, TAVR requires only one small incision in the groin into which a thin tube called a catheter is inserted. Cardiologists can insert replacement valves through this incision, threading them all the way into the heart via a blood vessel to replace old or malfunctioning valves (1). Its minimally invasive nature lends TAVR to be a more appealing option to surgery in several ways. While traditional open-heart surgery requires full sedation, patients receiving the TAVR procedure are lightly sedated but technically awake during the entire procedure. The average recovery time following TAVR is a few days, as opposed to the months of time required to fully recover from traditional surgery. What Happens During The Transcatheter Aortic Valve Replacement Procedure, 2019 (4) In the past, TAVR was reserved for the oldest and sickest patients, who doctors feared may not survive highly intense open-heart surgery. These recent trials, however, suggest that the procedure has been proven to be effective – and even preferable – in younger and healthier patients. Both clinical studies enrolled more than 1,000 patients, though they differed somewhat in design. The first tracked rates of death, stroke, and hospitalization at one year following both the open-heart and TAVR procedures. The results showed that TAVR yielded dramatically lower rates of adverse events: 8.5%, as compared to 15.1% for those who had open-heart surgery (2). The second study instead looked at the rates of death and stroke at two years post-procedure. This study similarly found that the rates of death and stroke were lower with the minimally invasive procedure: 5.3% for TAVR in comparison to 6.7% for open-heart surgery (1). Dr. Howard Herrmann, the director of interventional cardiology at The University of Pennsylvania, predicts that these studies’ results will “shift our thinking from asking who should get TAVR to why anyone should get surgery” (1). Similarly, Dr. Gilbert Tang, a heart surgeon at Icahn School of Medicine at Mount Sinai in New York, attests that he would choose TAVR if he were an eligible patient. Both of the clinical studies were sponsored by the only two companies that currently manufacture two different TAVR valves: Edwards Lifesciences, based in Irvine, California, and Medtronic, which is based in Dublin. Given the studies’ sponsors, several independent safety monitoring committees oversaw the trials in order to ensure that the results, which were also confirmed by independent statisticians, were accurately reported. The Edwards valve is compressed onto a balloon catheter, which is pushed through a blood vessel from the groin up to the aorta. Upon reaching the aorta, the balloon is inflated, which expands the new valve and pushes aside the failing valve. The Medtronic valve performs a slightly different function due to the fact that it is made of nitinol, a metal that expands with warmth and shrinks when cold. The chilled Medtronic valve is placed onto a catheter and freed upon reaching the aorta, where it is then warmed by the heat of the body and thus expands to fill the narrowed opening (1). Both of these valve procedures dramatically differ from that of open-heart surgery, during which doctors must removethe old valve prior to sewing in a new one. The results of these studies were presented this past month at the American College of Cardiology’s annual meeting, and will be published in The New England Journal of Medicine (2). The Food and Drug administration is expected to soon approve the procedure for lower-risk patients, which will make almost 20,000 more patients eligible for TAVR, in addition to the nearly 60,000 intermediate- and high-risk patients who currently receive this operation (1). The studies also have financial implications. Hospitals that offer the TAVR procedure will likely be negatively financially affected when lower-risk patients begin to request the procedure. This is because the TAVR valves are significantly more expensive than the surgically placed valves, yet insurers pay equally for either procedure, making open heart surgery the more profitable option (3). Furthermore, Edwards Lifesciences and Medtronic are the only two companies that currently manufacture the special valves, and Dr. Herrmann hopes that as the competition in this valve market increases in the coming years, the cost of the TAVR valves will eventually decrease (1). Although these studies do indicate that the TAVR leads to shorter recovery times and yields lower rates of post-procedure deaths and strokes, surgeons cannot yet determine whether the TAVR valves will last the 10 to 15 years expected of a replacement aortic valve (3). The average age of the subjects in these recent studies was in the low to mid 70s, which is a decade younger than most patients who were previously considered candidates for TAVR (1). Given that these trials have proven that younger patients will now be eligible for the procedure, it remains unclear whether TAVR fares better than traditional open-heart surgery in the long term. As for now, it seems as if patients, with the help of their cardiologists, will be the ones deciding which procedure they would rather receive. For more information, see [1] Kolata G. Tens of Thousands of Heart Patients May Not Need Open-Heart Surgery [Internet]. The New York Times. The New York Times; 2019 [cited 2019 Mar 31]. Available from: https://www.nytimes.com/2019/03/16/health/aortic-valve-replacement-heart.html.
[2] A Breakthrough in Heart Valve Surgery: More People Can Now Benefit from TAVR Procedure [Internet]. Columbia University Department of Surgery. 2019 [cited 2019 Mar 31]. Available from: http://columbiasurgery.org/news/2019/03/17/breakthrough-heart-valve-surgery-more-people-can-now-benefit-tavr-procedure. [3] MHVI Launches National Clinical Study of TAVR's Use in Low Risk Patients [Internet]. MedStar Heart & Vascular Institute. 2019 [cited 2019 Mar 31]. Available from: https://www.medstarheartinstitute.org/whychooseus/publications/mhvi-launches-national-clinical-study-of-tavrs-use-in-low-risk-patients/. [4] What Happens During The Transcatheter Aortic Valve Replacement Procedure. (2019). [image] [cited 2019 Mar 31] Available at: https://www.medtronic.com/us-en/patients/treatments-therapies/transcatheter-aortic-valve-replacement/about/tavr-procedure.html.
0 Comments
Leave a Reply. |