|
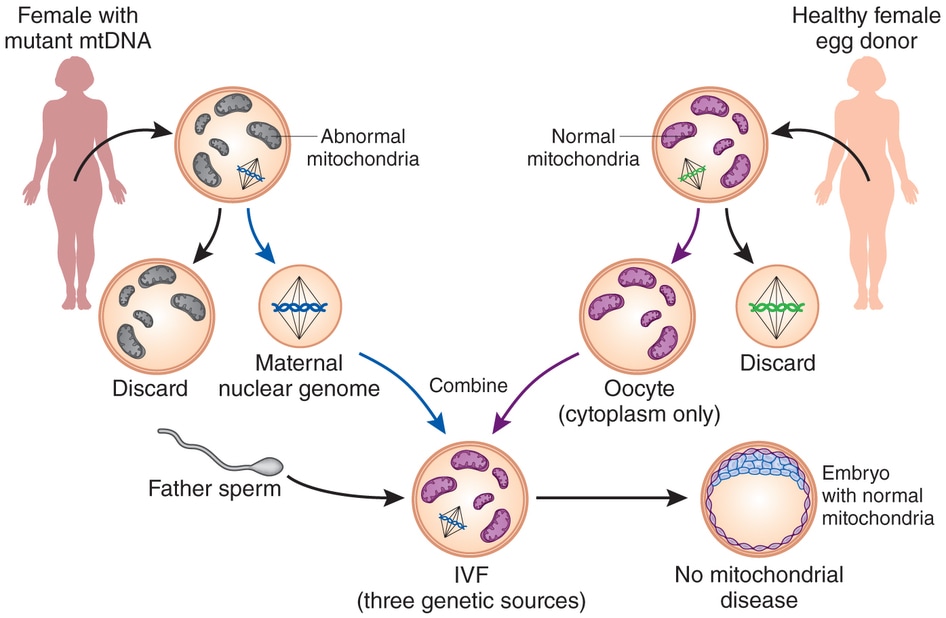
by Audrey Lee '16 and ScM'17 Each cell in the body contains two sets of DNA: nuclear DNA and mitochondrial DNA. DNA encodes essential information for cellular processes and overall survival, but can also be a powerful source of disease when mutated in certain ways. The set of DNA that comes from the nucleus serves as the information processing and administrative center of the cell. Image by Alex Pearlman Think of the nucleus as the CEO of an influential company, overseeing the company’s operations and directing its activity. The other smaller set of DNA comes from the mitochondria, which provide energy to sustain cellular processes. Within this company, the mitochondria are akin to the employees that provide the manpower to make sure the CEO’s directions are carried out. Whereas nuclear DNA is inherited from both parents, mitochondrial DNA is solely inherited from the mother. Through an unclear mechanism, mitochondria from the father’s sperm is either diluted or degraded [1]. As a result, mutations in the mother’s mitochondrial DNA are passed to her offspring, regardless of the offspring’s gender. Mutations in maternal mitochondrial DNA can lead to severe consequences even though DNA from the mitochondria only makes up a small portion of the total DNA. Mitochondrial replacement therapy (MRT), an innovative reproductive technology, was developed with the intent to prevent the inheritance of genetic disorders associated with mutations in mitochondrial DNA. Through this procedure, eggs that contain the mutated mitochondrial DNA from the diseased mother are removed from her body. In a laboratory setting, the diseased mitochondria are removed altogether, while still maintaining the DNA in the nucleus. Eggs require mitochondria to function, much like how powerful companies need their employees to succeed. The removed mitochondria are replaced by healthy mitochondria (and their associated healthy DNA) obtained from the eggs of a female donor. Following the replacement step, the egg from the affected mother no longer carries mutated mitochondrial DNA. This egg now holds nuclear DNA from the affected mother and healthy mitochondria from the healthy donor, and can be fertilized with sperm in a culture dish through a process called in vitro fertilization. The resulting healthy embryo is implanted back into the body of the original mother. The scientist sperheading this new research, Shoukhrat Mitalipov, Ph.D., is director of the Center of Embryonic Cell and Gene Therapy at Oregon and Science University. He believes that “this research suggests that we’re going to have the greatest success rates for producing an embryo free of disease-causing genetic mutations” [2]. Developing eggs from a patient carrying mitochondria with mtDNA mutations (gray) are replaced by mitochondria from a healthy egg donor (purple) [3]. Almost a year ago, a couple gave birth to a son using MRT. The mother carries genes for an inherited neurological disorder, Leigh syndrome in her mitochondria, resulting in the loss of the couple’s first two children. U.S. Congress prohibited the Food and Drug Administration from allowing MRT, forcing a New York City infertility doctor to travel to Mexico to perform the procedure [4]. Despite the success of MRT on this occasion, some scientists say that not enough research has been done to know whether it is safe. Paul Knoepfler, Ph.D., a cell biologist at the University of California, Davis explains “this is a troubling development on a number of levels. It could have gone wrong in any number of ways and still could” [5].
Aside from ethical concerns surrounding this controversial reproductive technology, there seems to be biological concerns that Mitalipov has brought to light. This past December, his research group published results illustrating the loss of healthy donor mitochondrial DNA over time. Unexpectedly, the miniscule amount of diseased mitochondrial DNA is capable of replicating and reinstating the presence of harmful mitochondrial DNA. In roughly 15% of cases, this mitochondrial reversion leads to fatal defects. These results demonstrate the biological consequences of replacing original mitochondrial genomes. If MRT continues in the future, then careful selection of donor mitochondria that share genetic similarities with the original maternal mitochondria will need to be put in place [6]. Along with the unnatural conception and birth of Louise Joy Brown through in vitro fertilization, the debate surrounding assisted reproductive technologies was also born. Since then, numerous procedures have continued to develop in order to address biological obstacles to natural reproduction. Included in these reproductive victories are intra-cytoplasmic sperm injection and in vitro gametogenesis, which address infertility. It is clear that progress in this field will continue, but it is important to fully understand the ethical and biological implications of these procedures before applying them to human beings. Sources:
0 Comments
Leave a Reply. |